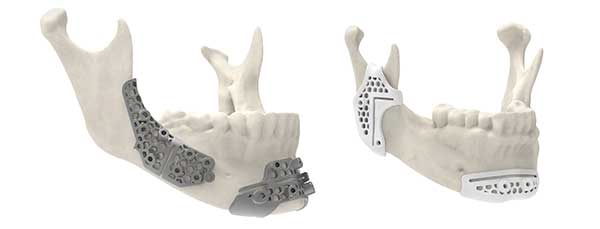
3D Systems has announced that the Food and Drug Administration (FDA) has provided 510(k) clearance for maxillofacial surgical guides 3D printed using its LaserForm Ti and DuraForm ProX PA materials. The new clearance allows for more surgical guide designs that improve performance and is the latest delivery on 3D Systems’ approach to address the needs of surgeons performing maxillofacial and reconstructive surgeries.
3D Systems’ surgical guides produced using these materials as part of its VSP (virtual surgical planning) System offer flexibility in design options, and are capable of having lower profile designs, while also possessing improved strength and rigidity.
3D Systems’ VSP technology received FDA market clearance as a service-based approach to personalized surgery, combining expertise in medical imaging, surgical simulation and 3D printing. The surgeon initiates the process, bringing their clinical knowledge and desired surgical plan to an online web meeting with a 3D Systems biomedical engineer to simulate and plan the surgical procedure. The outcome is a digital plan that is transferred to the operating room via 3D printed patient-specific models, guides, and templates. 3D Systems has provided VSP solutions or anatomical services in more than 120,000 patient cases.
“Through close collaboration with surgeons and Stryker’s CMF division, we’ve uncovered opportunities to refine VSP guide designs that leverage additional capabilities in our materials portfolio,” says Menno Ellis, executive vice president, healthcare solutions, 3D Systems. “Our expert biomedical engineers are now able to design surgical guides tailored to the surgeon’s needs with enhanced properties that can help improve accuracy and facilitate procedures in ways not previously possible.”
Improved Surgical Guide Designs
It is now possible to design surgical guides with less overall material bulk while improving strength and durability. Mechanical testing shows that Titanium cutting and marking guides produced in LaserForm Ti are stronger than traditional guides, according to 3D Systems. With the improvement in strength, Titanium fibula cutting guides can be thinner than traditional guides, facilitating improved access to the surgical site, the company adds.
Similarly, Nylon marking guides produced in DuraForm ProX PA exhibit higher toughness, rendering them better able to withstand forces applied during surgery, according to 3D Systems. This enables the creation of thinner guides than previously possible and facilitates a close, snap-like, fit-to-patient anatomy.
Flexibility in Treatment Options
With the new clearance, 3D Systems offers a portfolio of material and guide design options that provides surgeons with flexibility. The new materials allow for customizable designs, tailored to the cutting and drilling instrumentation to be used in surgery, to assist surgeons with accurately performing cutting and drilling operations, 3D Systems says.
Additionally, the guides created using LaserForm Ti and DuraForm ProX PA have been validated with a wider range of cleaning and steam sterilization options. Users now have more flexibility in using manual or automated cleaning methods, and the devices can be steam sterilized using a wrap or pouch under a wider range of accepted sterilization cycles.
Sources: Press materials received from the company and additional information gleaned from the company’s website.
About the Author
Press releases may be sent to them via [email protected]. Follow Robotics 24/7 on Facebook
Follow Robotics 24/7 on Linkedin
Article topics
Email Sign Up