From fitness monitors to advanced hearing aids, sensors and wearable glucose monitors, more people than ever rely on wearable medical devices. Designing these devices presents the challenge of integrating the various elements inside one device—and then that device must fit and be inconspicuous for various users.
Wearable medical environments are often harsh. So, packaging plays a key part; mechanical and electrical/electronic requirements are equally important, and must be protected.
Informational flow is also a key issue, as such devices require bidirectional data streams.
“This type of device is extremely challenging to design, and extremely iterative in nature,” says Stephen Endersby, director of product portfolio management at SolidWorks, Dassault Systèmes. “The ECAD people are fighting the industrial designers, and the poor mechanical guys are caught in the middle trying to keep everybody happy.”
The engineers on such projects “hold the whip,” adds Endersby. “If it isn’t functional it won’t sell.” Despite that, Endersby says, “the overwhelming design concern in wearable medical devices mostly comes down to packaging.”
Four types of devices
Engineering literature shows there are four primary types of wearable medical devices. The first is passive monitors that only record data. Second is surveillance devices, which record and report data either on a schedule or in real time. The third is diagnostic devices, which are capable of making decisions such as when to alert the user or to “call home” to medical professionals. The fourth is therapeutic devices—the most complicated of the four—which treat the condition.
Diagnostic and therapeutic devices come with higher legal risk and more lengthy regulatory review cycles.
Wearable medical device design is “a huge iterative process,” notes Endersby. “Industrial design and ECAD are packaged up into a version, then you start hammering on it for product performance in terms of simulations. EMC [electromagnetic compatibility], electromagnetics and antenna design and heat/shock/thermal are all part of it.”
Five principles for design
Simbex is an engineering consulting firm that specializes in wearable medical devices. The firm works from five key principles:
Establish context for all collected data: Make sure to use as much readily available information as possible, including external sources such as calendar integration, proximity tracking and location services.
Do initial prototyping with existing solutions: Create an early proof-of-concept prototype using existing sensor solutions before specifying a custom sensor. It helps the budget and provides a better understanding of sensor requirements.
Algorithm development should describe system dynamics: “Accurately predicting behavior and generating observations from sensor measurements requires algorithms which can describe underlying system dynamics,” notes a statement of principles on the company’s website. “Power budget and user experience are key drivers for user engagement.”
Battery life and sensor optimization go hand in hand: Consumers have high expectations regarding battery life, and these two are key in the device’s power budget.
Design for manufacturability: Simbex says it incorporates manufacturing processes into product design from the start, new and established processes.
Starfish Medical is another engineering firm that focuses on wearable medical devices. It follows five rules in initial design.
- Research the user population first. Define use scenarios and workflows that match current protocols.
- Make sure there is clear and open communications between industrial design and the electrical/electronic engineers.
- Determine early if the device is to be disposable or reusable.
- Be careful if working with adhesive devices; there is no such thing as an adhesive suitable for all skin-work devices.
- Thoroughly test all external components for biocompatibility.
There is a difference between a health device and a medical device, says Endersby.
“Take an insulin pump,” he says. “It’s not real high tech but it must deliver a set amount of the drug at a set time and a set rate. The medical impact of something going wrong with a drug delivery device is an order of magnitude worse than a health device losing your jogging data.”
The requirements of medical devices for traceability, approvals, testing and more “make the importance of the collaboration platform to be much more stringent,” he adds.
Establishing the paper trail
“Engineering teams in this field often spend more time on the paper trail and documentation than they spend on actual product design,” notes Benjamin Jordan, senior product manager, Fusion 360 Electronics at Autodesk. “Accordingly, anything that helps shorten those tedious design review and compliance cycles brings great value.”
Autodesk is building out a collaboration platform based on its Fusion 360 portfolio. The company sees such a collaboration platform as a key to success in medical device design, which requires the input of multiple engineering disciplines, plus product managers, specification authors, standards compliance test engineers and executive and government officials.
“This is where a robust product management system pays off. With all these solutions under one roof, the design process becomes more efficient and costly designs are avoided,” says Jorge Garcia, Fusion 360 Technical Specialist, Electronics, for Autodesk.
Garcia describes a typical workflow in which a design team starts with the wearable’s form factor. The mechanical and industrial designers collaborate to model that form factor in the mechanical environment. From there the electronics engineer derives the printed circuit boards (PCB) outline and gets to work on the layout.
If the mechanical team later realizes something about the enclosure needs to change, that team can make the required change and the electronics engineer can then pull in that change downstream. As the design develops, engineers can perform stress and thermal simulations on the PCB within the enclosure, and uncover aspects of the design that need to be adjusted and optimized for cooling or durability.
“Teams that win the market race are teams with the ability to iterate quickly, remaining agile and flexible when one change to a project requires that other, accommodating changes, be made easily,” says Garcia.
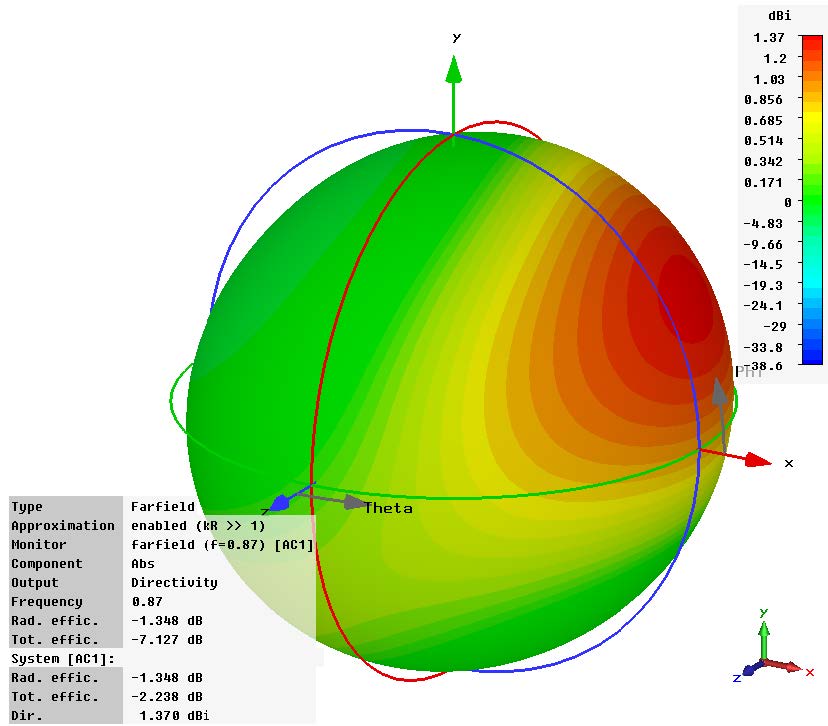
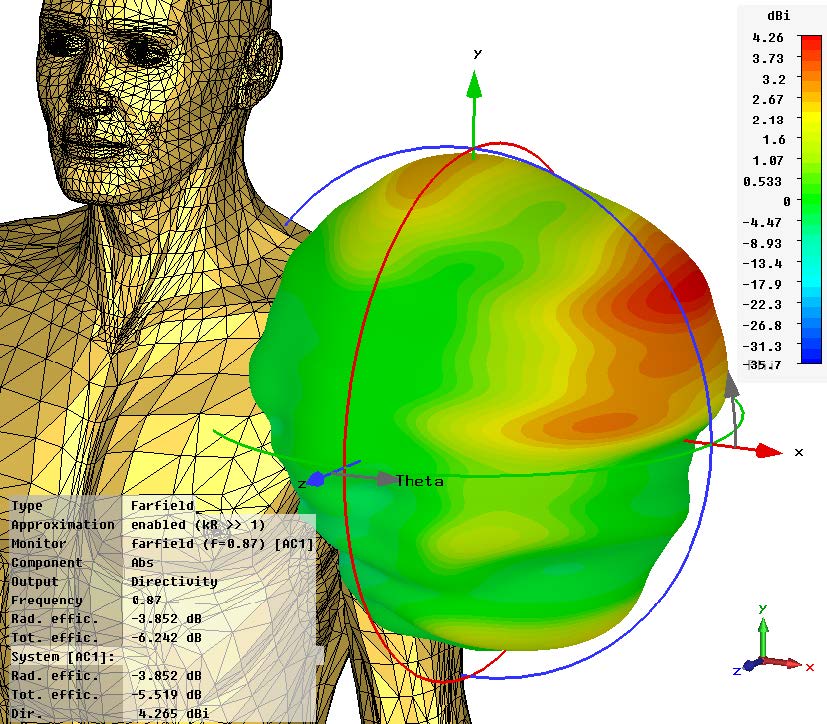
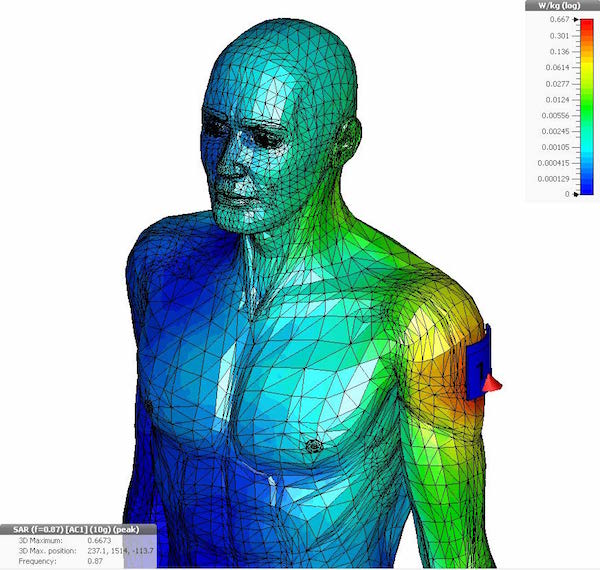
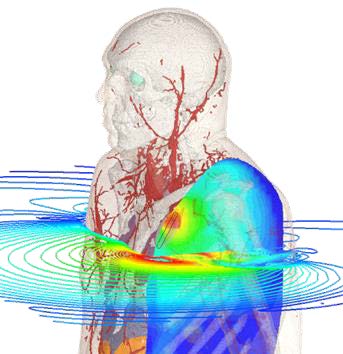
Sequence of a simulation of a wearable medical device conducted in Dassault Systèmes Simulia. Images courtesy of Dassault Systèmes.
What about simulation?
Garcia notes that historically, simulation is a polarizing subject. “Some engineers completely dismiss it while others treat it as the holy grail of engineering. As usual, reality lies somewhere in the middle.”
To leverage simulation effectively, Garcia identifies two typical prerequisites. First, the study needs to be set up correctly, including accurate models and proper boundary conditions. Second, the engineer or designer must have an expectation of what the result should be.
“This last point is very important,” says Garcia. “If there’s no preliminary expectation of what the simulation should produce, there’s no way to make sure the first point is satisfied.”
Setting up a simulation correctly is not a trivial task, Garcia says, so if users expect the simulator to synthesize a solution, “it often leads to disappointment and the polarized attitude toward simulation in the engineering community.”
Yet, simulation is a vital tool to medical device design.
“Anything that needs to interface with the human body must be safe,” Garcia notes.
Thermal simulations can help verify that a device won’t burn the person using it. A simulation program with integration circuit emphasis (SPICE) analysis can verify the soundness of a design and make sure that all voltage and current levels are within safe limits.
“Simulation can add significantly to the documentation provided as evidence for standards compliance and certification,” says Jordan.
Dassault Systèmes’ Endersby believes the collaboration platform approach to product design is especially helpful for simulation.
“Companies who need to develop these tools all have the same challenges—packaging, power consumption, EMC and more. If things go wrong, oops!”
The traditional siloed approach is just too inefficient, Endersby notes.
“If the mechanical guys are working in one system and they pass a neutral format to electrical, and then electrical does the same to simulation, it doesn’t work.”
A common data language is required. “Antennas need a common data model and a common language,” says Endersby. “So when mechanical and electrical are set up, they can take to simulation.”
The main goal is about the pain-free exchange of information between hardware, software, mechanical, design and prototyping.
“All that information must be as frictionless as possible. Where there is friction there is area for mistakes. We believe the platform approach is where companies will start to win,” Endersby says.
Randall S. Newton is principal analyst at Consilia Vektor, covering engineering technology. He has been part of the computer graphics industry in a variety of roles since 1985. Contact him at [email protected].
Article topics
Email Sign Up


















